Injectible immune modulator drug data from 6 sequential pet dogs with spontaneous, mostly recurrent mast cell cancers
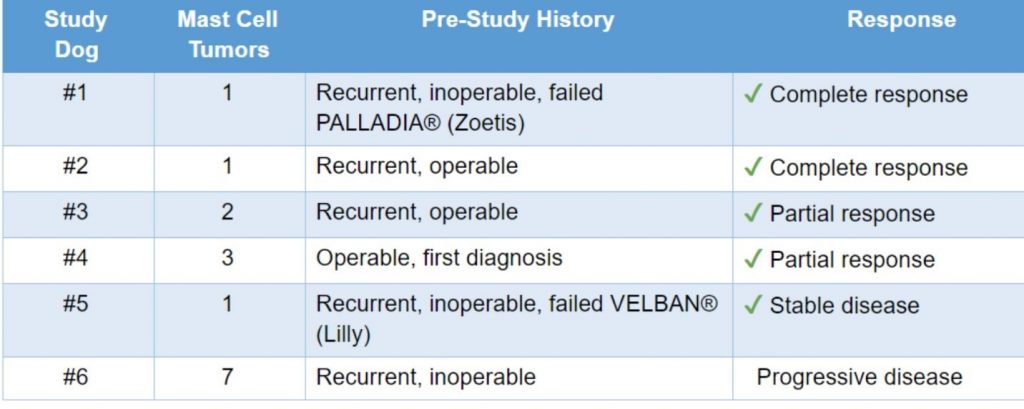
“Complete response” was defined as the disappearance of the tumor and absence of recurrent disease within a month after the initial confirmation of complete response
“Partial response” was defined as at least a 30% decrease in the sum of the longest diameter of tumors.
“Progressive disease” was defined as at least a 20% increase in the sum of the longest diameter of tumors.
“Stable disease” was defined as neither partial response nor progressive disease.
These are the standard FDA criteria used in clinical trials.
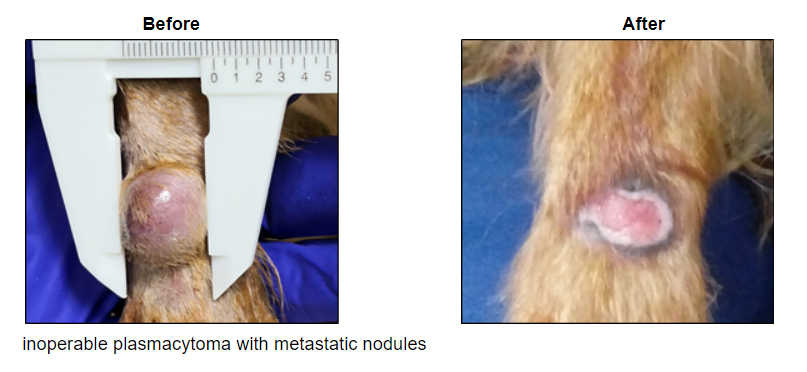
Our injectable cancer therapies are showing promising immune results in real cancers in pet dogs. The strategy is to reverse tumor-induced immune suppression, thus making “cold” tumors “hot” to the immune system. In human patients these aim to increase responsiveness to checkpoint inhibitors, which currently fail in the majority of patients and can cause severe side effects in patients who do respond.
In dogs with melanoma, we are using a combination of (i) our original intra-tumor cisplatin drug to induce neoantigens, and (ii) our new immunotherapy drug to attract and drive an attack by the dog’s natural immune system. Notably, our platinum drug formulation appears to be showing immunogenic cell death, including calreticulin translocation, a known indicator of immunological cell death.
Intra-tumor cisplatin drug graphic:
Key attributes of the re-formulated intra-tumor cisplatin drug
- Size: The 20nm size allows for penetration into tumors and retention in adjacent lymph nodes where cancers metastasize.
- Drug: This platform allows packaging of systemically toxic chemotherapeutics into a nanoparticle that can be locally delivered with minimal local toxicity. Cisplatin, doxorubicin, and docetaxol have been tested with encouraging results. The platform is generalizable to a broader list of agents over a wide molecular weight range (up to small antibodies).
- Hyaluronan conjugation allows local injection directly into the tumor, and also targets a key cancer receptor, CD44. Higher CD44 expression is associated with aggression and with so-called “cancer stem cell” status.
- Calteticulin translocation has been noted, consistent with immunogenic cell death
 |
Transmission electron microscopy picture showing HA and cisplatin condense into a <100 nm particle. |
Drug travels from injection site into local lymph nodes
Efficacy in Murine Xenograft of human head and neck squamous cell carcinoma (MDA1986)
Animals received 3 doses (1/week) once tumors researched 50 mm2. All drugs/controls (n=6-8/group) dosed at 3 mg/kg on a platinum basis.
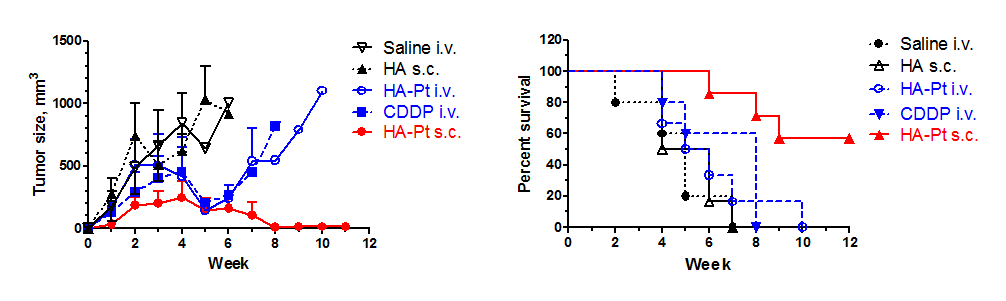
Efficacy in Murine Xenograft of breast cancer (locoregional with lymphatic mets, MDA-MB-468LN)Animals received 3 doses (1/wk) once tumors researched 100 mm2. All drugs/controls (n=6-8/group) dosed at 2 mg/kg on a doxorubicin basis.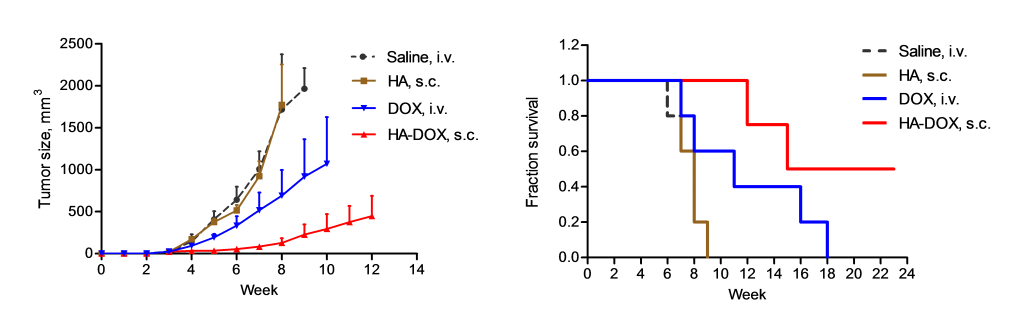 Canine Pharmacokinetics 96 hours after a single 10 mg/kg injection of HylaPlatCanines presented with spontaneous leg sarcomas. After 96 hrs, legs were amputated and drug levels in the tumor and sentinel lymph node were determined by ICP-MS. I.v. data were taken from historic files at Colorado State University.
Canine Pharmacokinetics 96 hours after a single 10 mg/kg injection of HylaPlatCanines presented with spontaneous leg sarcomas. After 96 hrs, legs were amputated and drug levels in the tumor and sentinel lymph node were determined by ICP-MS. I.v. data were taken from historic files at Colorado State University.
 |
| Parameters | Cisplatin NPs Intra-tumoral |
Cisplatin intravenous |
| Cmax, ng/mL | 81.6 ± 40.4 | 1793.8 ± 359 |
| t1/2, hrs | 51.2 ± 29.1 | 0.49 ± 0.03 |
| AUC0-inf, ng·hr/mL | 3562.1 ± 2031.1 | 1240 ± 200 |
| Tumor, ng/g at 96 hrs | 5650.0 (3324.5 – 8228.8) | – |
| Lymph node draining tumor, ng/g at 96 hrs | 2485.0 (129.5 – 6066.0) | 5 |
Data from Dr. Deanna Worley, DVM, Colorado State University. Am. J. Vet. Res. 73(12):1969-76 (2012).